Nanodiamond‐Based Separators for Supercapacitors Realized on Paper Substrates
Preview Image: 123dartist/Shutterstock
Introduction
Cellulose-based substrates are considered among the most appealing solutions for green recyclable flexible electronics due to their compatibility with large-scale printing techniques. In that context, cellulose nanofibrils networks ensure a high surface area and a large amount of hydroxyl groups that can be functionalized, tuned, and are compatible with hydrophilic environment.[1]
In the current work, a route for the realization and characterization of a biocompatible paper-based symmetric supercapacitor using electronic paper and common copy paper as substrates and poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS) spray-coated electrodes is proposed. Moreover, we proposed a composite material based on hydroxypropyl cellulose (HPC) and detonation nanodiamond (DND) as separator and as solid electrolyte. DNDs are obtained by the purification of the soot generated through the detonation of explosives and present outstanding properties, such as thermal conductivity, thermal and chemical stability, low electrical conductivity, and mechanical properties (abrasiveness and hardness).[2] Of particular interest, it has been shown that porous aggregates of DND and of DND dispersed in a polyelectrolyte (Nafion) are capable of increasing the ionic conductivity in the electrochemical system.[3, 4]
We also compared the performance of devices based on solid electrolyte (HPC + DNDs) and liquid solution electrolyte (HPC + ND + Na2SO4) realized on both substrates (copy and electronic paper) using PEDOT:PSS spray-coated electrodes. The performances obtained are comparable with those found in the literature for devices realized on polyethylene terephthalate substrate using spray-coated PEDOT:PSS electrodes.[5]
Methods
To investigate the effects of different paper morphologies on the supercapacitor, we used a common acid-free copy paper with alkaline reserve (copy paper) and an electronic ultra-smooth paper designed for high-definition patterning and electroplating (electronic paper). The negative electrode was deposited on paper substrates by spray-coating. A thickness of 400 nm of PEDOT:PSS was obtained. After the deposition of the separator and the solid electrolyte, the positive electrode was spray-coated. The thickness of 300 nm of PEDOT:PSS for the positive electrode was chosen to avoid the melting of the separator, but still maintaining a sheet resistance comparable with the negative electrode. The deposition of the separator and the solid electrolyte was conducted by an automatized blade/slot-die coater with drying step (hot air).
Morphology characterization of the devices’ layers was performed using an Olympus LEXT OLS4000 laser microscope and a field emission SEM. The thickness of the layers was evaluated using a feeler gauge (digital thickness gauge).
Electrical and electrochemical characterizations were made using a four-probe setup connected to a source meter (Keithley 2420), and a potentiostat PGSTAT302N (EcoChemie Autolab B.V.).
Results and Discussion
The steps of fabrication and the final layout of the device can be seen in Figure 1.
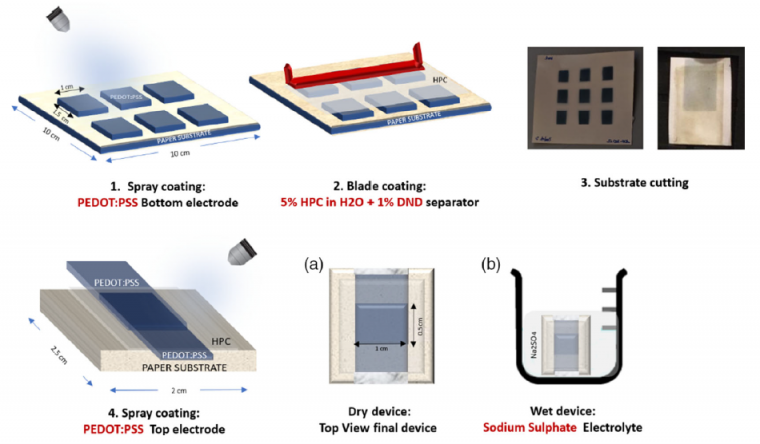
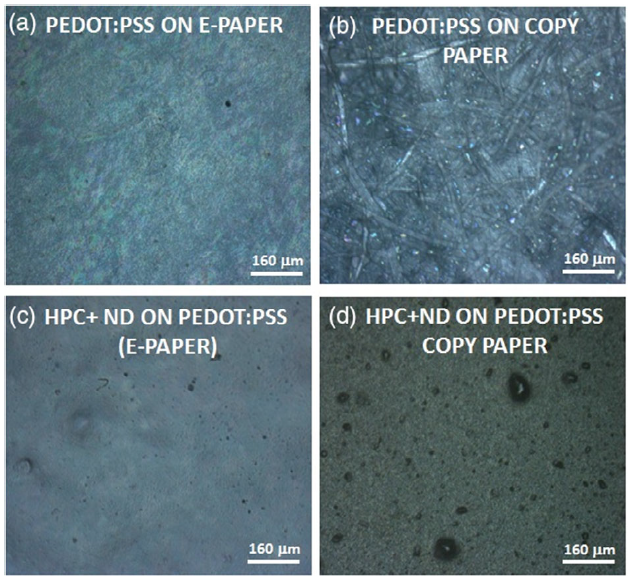
Figure 2 shows the morphology of the spray-coated PEDOT:PSS negative electrode deposited on electronic and copy paper. Here, it is possible to observe that the porous and rough nature of the copy paper substrate affects the deposition of the PEDOT:PSS electrode. Comparing the morphology of the HPC + DND separator deposited on the PEDOT:PSS electrodes, it can be noted that a smoother surface can be obtained on electronic paper, whereas for copy paper, the roughness of the starting PEDOT:PSS electrodes affects the separator morphology.
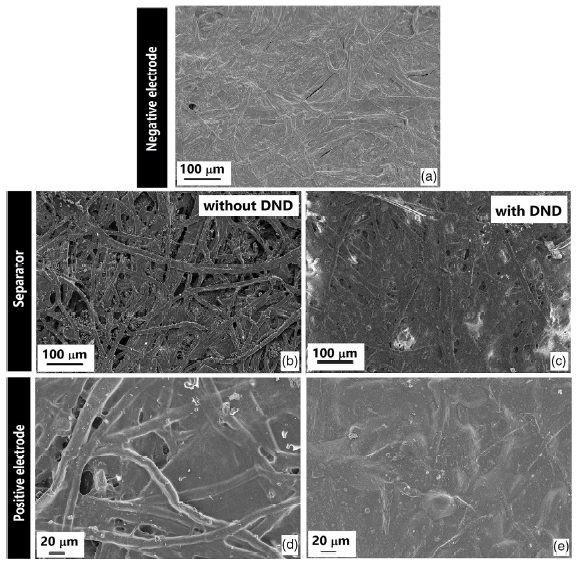
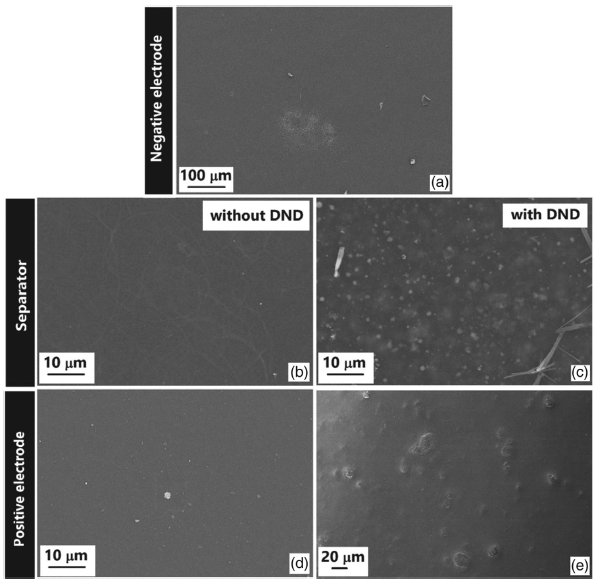
Figures 3 and 4 show layer-by-layer scanning electron microscopy (SEM) images, from the negative electrode to the subsequent upper layers of the final wet device on copy and electronic paper, respectively. On copy paper, the presence of DND seems to strongly affect the substrate roughness, filling the concavities and voids in the paper structure (Figure 3c), thus allowing a better deposition of the positive electrode (Figure 3e), which results in a more continuous and homogenous appearance with respect to the device prepared without DND (Figure 3d). On the contrary, as expected, on electronic paper, a smoother surface is always observed. PEDOT:PSS deposition results a high amount of homogeneity for both electrodes (Figure 4a,d). Conversely to what we observed with copy paper, the presence of DND leads to a rougher surface (Figure 4c,e), without, however, affecting the homogeneity of the deposition. A few Na2SO4 electrolyte crystals were observed, especially in the case of electronic paper, where the presence of DND seems to increase the quantity of the electrolyte retained in the separator.
Electrochemical tests of the assembled devices with both the typologies of electrolyte and electrode substrate show only an electric double-layer capacitor (EDLC) behavior. A stronger resistive behavior is produced for solution electrolyte and was observed in charge-discharge tests. We also identified a better charge propagation at lower scan rates. In fact, we observed an increasing shape deviation with an increase in the scan rate, probably due to higher internal resistance. In all the cases, we did not record rectangular-shaped cyclic voltammograms due to the use of PEDOT as electrode material.[6]
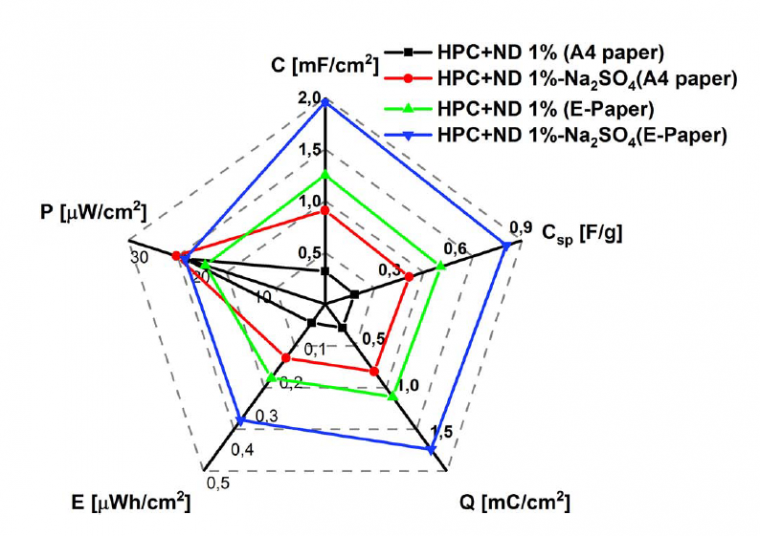
Supercapacitor parameters were calculated from charge–discharge cycles, are shown in Table 1, and are graphically represented in Figure 5. It is possible to observe that the devices produced with electronic paper show higher values of specific capacitance for both solid and solution electrolytes. Conversely, comparable power densities were achieved for both papers in dry and wet supercapacitors.
Table 1. Electrochemical parameters extracted from charge-discharge cycles.
HPC + ND 1% solid electrolyte | C | Csp [F g-1] | Q | E [µWh cm-2] | P [µW cm-2] |
Copy paper | 319.14 | 0.13 | 255.31 | 0.056 | 22.69 |
E-paper | 1248.09 | 0.52 | 998.47 | 0.221 | 19.48 |
HPC + ND 1% + Na2SO4 |
|
|
|
|
|
Copy paper | 907.37 | 0.38 | 725.89 | 0.161 | 24.19 |
E-paper | 1957.29 | 0.82 | 1565.83 | 0.347 | 22.77 |
Although still an object of study, it is reasonable to assume that the hygroscopic environment guaranteed by the cellulose derivative-based separator allows the deprotonation/protonation of carboxylates and amphoteric functional groups present on the DND surface. As hypothesized by Postnov et al. (2017) and Gareeva et al. (2014),[3, 4] the presence of DND could also ensure a structural arrangement of ionic channels and pores of DND aggregates and HPC chains organization crucial to charge transfers.
In Figure 8, the normalized capacitance values decay obtained from several charge-discharge cycles for both solid (Figure 8a) and solution electrolytes (Figure 8b) are reported. Devices on electronic paper present much higher capacitance values, and the capacitors realized with a solid electrolyte exhibit higher capacitance retention. From this experimental data, we assume that in absence of DND, it is not possible to have significant measures due to observed short circuit of the device. This is ascribable to the deterioration (due to hydrolysis processes) of the thin separator layer, mainly caused by the high acidity of PEDOT electrodes above and below it. The better performance of the device with the solid electrolyte (DND + HPC) if compared with the one with solution electrolyte (DND + HPC + Na2SO4) could also be related to the deterioration of the HPC layer that occurs when the device is immersed in the electrolyte solution. Therefore, it is clear that DND manages to preserve the integrity of the separator; however, a significant drop in potential was still observed in the charge–discharge tests of the wet device if compared with the solid-state electrolyte curves.
Conclusion
We fabricated symmetric supercapacitors using upscalable techniques and green materials. Our devices are based on conducting PEDOT:PSS polymer electrode sprayed on two different types of papers (i.e., copy paper and electronic paper). Moreover, a new nanocomposite material based on HPC charged with DNDs was used as both separator and solid electrolyte and compared with the same composite wet with a Na2SO4 solution electrolyte. This work confirmed DND electrolytic properties due to both its special surface chemistry and its capability to modify and organize the structure of the matrix in which it is dispersed. Moreover, DND has once again proven to be an efficient consolidating material for cellulose derivatives, as we demonstrated that the device does not work in its absence due to the chemical nature of the separator.
The performance of our devices has been evaluated, and maximum power densities of ~24 μW cm-2 and specific capacitance of ~0.8 F g-1 were achieved. Our results demonstrate that it is possible to obtain comparable performances on paper substrates with respect to analogous supercapacitors fabricated on plastic substrates with spray-coated PEDOT:PSS electrodes.
Literature
[1] Z. Wang, P. Tammela, M. Strømme, L. Nyholm, Adv. Energy Mater. 2017, 7, 1700130.
[2] V. N. Mochalin, O. Shenderova, D. Ho, Y. Gogotsi, Nat. Nanotechnol. 2012, 7, 11.
[3] V. N. Postnov, O. V Rodinkov, L. N. Moskvin, A. G. Novikov, A. S. Bugaichenko, O. A. Krokhina, Russ. J. Gen. Chem. 2017, 87, 2754.
[4] F. Gareeva, N. Petrova, O. Shenderova, A. Zhukov, Colloids Surfaces A Physicochem. Eng. Asp. 2014, 440, 202.
[5] C. J. Zhang, T. M. Higgins, S. H. Park, S. E. O’Brien, D. Long, J. N. Coleman, V. Nicolosi, Nano Energy 2016, 28, 495.
[6] P. Kumar, Z. Yi, S. Zhang, A. Sekar, F. Soavi, F. Cicoira, Appl. Phys. Lett. 2015, 107, 053303.
Original Publication
G. Polino, A. Scaramella, V. Manca, E. Palmieri, E. Tamburri, S. Orlanducci, F. Brunetti, Energy Technol. 2020, 8, 1901233. DOI: 10.1002/ente.201901233