Improvement of the Electrochemical Behavior of (Sb, Sn, Cu)O Ceramic Electrodes as Electrochemical Advanced Oxidation Anodes
Abstract
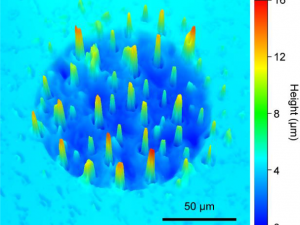
This work explores the possibility of increasing the active surface of a Sb‐doped SnO2 ceramic electrode using CuO as sintering aid, by incorporating petroleum coke as a pore generator. In order to fulfil this goal, three series of (Sb, Sn, Cu)O electrodes with different coke contents were synthetized. The properties of the electrodes, and their microstructure, change significantly as a function of the coke content before sintering. The electrochemical characterization of the synthesized electrodes showed that the coke addition before sintering causes two antagonist effects on the performance of the (Sn, Sb, Cu)O as anodes in electrochemical advanced oxidation processes (EAOP). On one hand, it significantly improves the electrochemical roughness factor of the electrode, solving the densification problem in this way. On the other hand, it worsens the electrochemical behavior of the electrode: narrowing its electrochemical window; and “activating” it slightly. The addition of coke before sintering changes the kinetic parameters, leading to a kinetic situation in which the accumulation of hydroxyl radicals is slightly lower. A balance must be sought: an intermediate coke content will improve significantly the electrochemical roughness factor of the electrode, but will only worsen slightly its electrochemical behavior, leading to an optimum (Sn, Sb, Cu)O EAOP anode.
Full article:
Source: Preview Image: rolkadd/Shutterstock