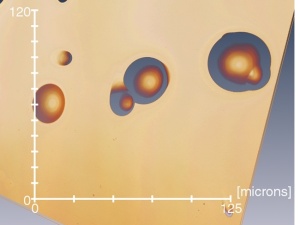
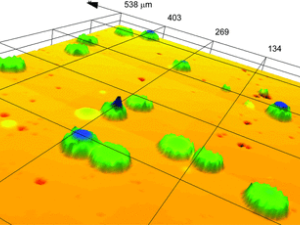
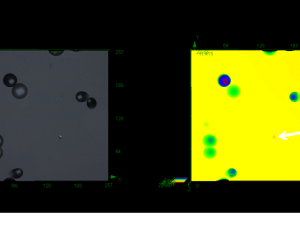
Particles: Diverse Properties, Uncountable Applications
Unique properties
Nano and microparticles are distinctive materials that have enormous technological and scientific value. They are used in many applications, including energy, medical, and environmental. Even though nano and microparticles have the same composition as analogous bulk material, they display very interesting optical, electrical, thermal, and magnetic properties. Materials science has developed several methods to tune these properties for specific applications.
Nano and microscale particles have two technologically relevant features. First, particulate systems have a very high surface-to-weight ratio. As a result, surface energies are large with respect to volumes, and, therefore, the energetics of reactions (and rates) are different. This characteristic makes them valuable for a wide range of applications such as coatings, catalysts, adsorbents, and more. On the other hand, when the scale is about a few nanometers, new properties and new striking phenomena take place.
Properties such as melting point, fluorescence, electrical conductivity, magnetic permeability, and chemical reactivity change as a function of the particle’s size.[2] For example, at the nanoscale, quantum effects are dominant, which is why semiconductor quantum dots emit different wavelengths of light depending on their nano-size[3]. Another example is magnesium (Mg), where the number of atoms and the size of the cluster/particle, determines the electronic band gap of the material. The electronic properties change from a semi-conductor (<18 atoms) to metallic when increasing the size.[4]
Nanoparticles made of gold and silver exhibit size-dependence in their optical properties.[6] Gold and silver nanoparticles absorb and scatter light very efficiently. They interact strongly with light because the conductive electrons on the metal surface undergo a collective oscillation when they are excited by light at specific wavelengths; this is known as surface plasmon resonance (SPR). The absorption and scattering properties of these nanoparticles can be tuned by controlling the particle size, shape, and the local refractive index near the particle surface.[6] Gold and silver nanoparticles have diverse applications in drug delivery, such as determining and sensing drugs in pharmaceuticals.[7]
Diverse shapes and synthesis paths
Based on their shape, nanoparticles can occur as nanosheets or nanofilms, which have at least one dimension in this size range; and nanorods and nanoparticles, which have two and three dimensions in this size range, respectively. There are also nanotubes, which are nanoscale materials that have a tube-like structure (e.g., carbon nanotubes).
Nano and microparticles are usually synthesized using physical and chemical methods. In physical methods, particles are generated by decreasing the size of the source material (top-down approach). Physical techniques include milling, gas condensation, electro-spraying, lithography, and thermal decomposition. On the other hand, in chemical methods, particles are created by nucleating and growing particles from atomic or molecular precursors in the liquid or vapor phase of a chemical reaction (bottom-up approach). Chemical methods include microemulsion, emulsion polymerization, hydrothermal, microfluidic, chemical vapor, pyrolysis, and sol-gel processes. Chemical methods generate nanostructures with fewer defects, enable more complex and homogeneous compositions, and are easily scalable for low-cost and rapid fabrication.
Uncountable applications
Several industries benefit from nano and microparticles. The microelectronics industry—one of the first to use nanoparticles—supplies an annual market of 500,000 million dollars (2016).[9] Today, medicine is one of the most promising areas for the application of microparticles to treat specific diseases, as drug delivery systems and/or nano/microactuators.[10] Moreover, in the field of energy, microparticles are being used to develop systems with greater energy storage capacity, as in the case of carbon nano tubes in modern batteries.[9] Likewise, energy capture capacity can lead to the development of devices that produce their own energy with a significant advantage over wiring or batteries. The food industry also sees advantages in the use of nanoparticles, since they can be used to detect changes in food at early stages, helping avoid contamination prior to consumption.[9]
As our understanding of how we can develop and improve the synthesis and characterization methods of nano and microparticles, we will continue to find new applications that help solve global challenges in applications like energy, health, and food, positively impacting our daily lives thanks to their fascinating and tunable properties.
References
[1] A.C. Grimsdale, K. Müllen, The Chemistry of Organic Nanomaterials, Angew. Chemie Int. Ed. 44 (2005) 5592–5629. https://doi.org/10.1002/anie.200500805.
[2] K. Yoshihara, M. Sakamoto, H. Tamamitsu, M. Arakawa, K. Saitow, Extraordinary Field Enhancement of TiO 2 Porous Layer up to 500‐Fold, Adv. Opt. Mater. 6 (2018) 1800462. https://doi.org/10.1002/adom.201800462.
[3] D. Ren, B. Wang, C. Hu, Z. You, Quantum dot probes for cellular analysis, Anal. Methods. 9 (2017) 2621–2632. https://doi.org/10.1039/C7AY00018A.
[4] O.C. Thomas, W. Zheng, S. Xu, K.H. Bowen, Onset of Metallic Behavior in Magnesium Clusters, Phys. Rev. Lett. 89 (2002) 213403. https://doi.org/10.1103/PhysRevLett.89.213403.
[5] Introduction to Nanoscience: Some Basics, (n.d.). https://serc.carleton.edu/msu_nanotech/nano_intro.html.
[6] A.J. Haes, C.L. Haynes, A.D. McFarland, G.C. Schatz, R.P. Van Duyne, S. Zou, Plasmonic Materials for Surface-Enhanced Sensing and Spectroscopy, MRS Bull. 30 (2005) 368–375. https://doi.org/10.1557/mrs2005.100.
[7] K. Alaqad, T.A. Saleh, Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs, J. Environ. Anal. Toxicol. 6 (2016). https://doi.org/10.4172/2161-0525.1000384.
[8] C.A. Mirkin, The Beginning of a Small Revolution, Small. 1 (2004) 14–16. https://doi.org/10.1002/smll.200400092.
[9] C.R. Kagan, L.E. Fernandez, Y. Gogotsi, P.T. Hammond, M.C. Hersam, A.E. Nel, R.M. Penner, C.G. Willson, P.S. Weiss, Nano Day: Celebrating the Next Decade of Nanoscience and Nanotechnology, ACS Nano. 10 (2016) 9093–9103. https://doi.org/10.1021/acsnano.6b06655.
[10] D. Ditter, P. Blümler, B. Klöckner, J. Hilgert, R. Zentel, Microfluidic Synthesis of Liquid Crystalline Elastomer Particle Transport Systems which Can Be Remote‐Controlled Magnetically, Adv. Funct. Mater. 29 (2019) 1902454. https://doi.org/10.1002/adfm.201902454.
Source: Preview Image: Floriana/Getty Images