Correlative Extinction and Single Fluorophore Bleaching Microscopy for Ligand Quantification on Gold Nanoparticles
Abstract
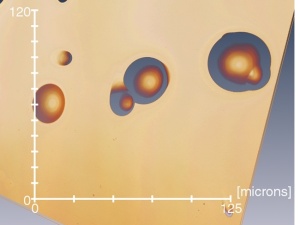
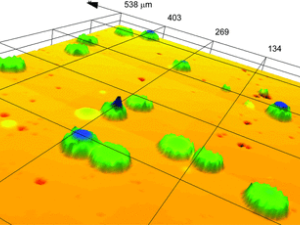
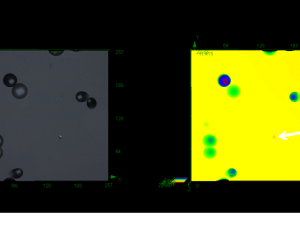
Nanoparticles (NPs) are promising therapeutic delivery agents, with the number and manner of presentation of cell-binding ligands on the NP affecting the eventual fate of the therapeutic. Whenever NPs are conjugated with biomolecules, a heterogeneous population of decorated NPs will be produced and these subpopulations of particle-ligand structures need to be characterized for a reliable interpretation of NP-based data. An optical microscopy method is reported to quantitatively evaluate the conjugation on a single-particle basis in samples consisting of gold NPs (GNPs) decorated with holo-transferrin fluorescently labeled with Alexa647 (Tf). Widefield fluorescence and extinction microscopy are employed on NP-ligand constructs, alongside a correlative analysis that spatially co-localizes diffraction-limited sources of fluorescence with the optical extinction by individual GNPs. A photobleaching step analysis estimates the number of fluorophores contributing to the detected emission rate. The method quantifies the number of fluorescent biomolecules attached per GNP, the numbers of unconjugated GNPs and unbound Tf present within the mixed population, and the size and intraparticle clustering propensity of conjugated GNPs. A high variability is found in the number of Tf ligands per GNP within the GNP population, when analyzed at the single-particle level, unraveling a non-trivial statistical distribution not accessible in ensemble-averaged approaches.