Effect of Surface Volta Potential of Cr-containing Steel on Uniform Corrosion and Pitting Corrosion
Abstract
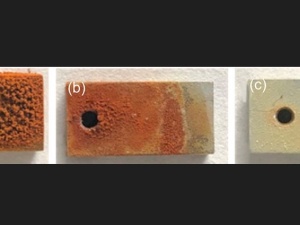
The corrosion behavior of 5.5%Cr–8.5%Cr steels in a CO2 environment was studied. To clarify the effect of Volta potential on uniform corrosion and pitting corrosion, weight loss test, electrochemical measurement, scanning electron microscope, energy dispersive spectrometer, confocal laser scanning microscope, and scanning Kelvin probe force microscope were conducted. The results show that there is a linear relationship between the surface potential of the steel substrate and the self-corrosion potential. The increase of the Cr content reduces the surface potential and increases the self-corrosion potential, reducing the thermodynamic tendency of corrosion. The Al-oxide inclusions show lower potential than the substrate, and their sensitivity to pitting corrosion is affected by the potential difference with the substrate. The larger the potential difference, the easier it is tantamount to induce pitting corrosion.
Full article:
Source: Preview Image: Jason A. Wright/Shutterstock